In the early days of rattlesnake antivenom, it was not uncommon for a doctor to say that the treatment could be worse than the bite.
The problem stemmed from a crude production process that extracted “polyclonal” antibodies (i.e. whole immunoglobin G) from the blood of a host animal. By definition, these antibodies were actually a mix of different antibodies: while some were generated as part of the animal’s natural immune response to a venom antigen (and therefore combatted envenomation), others were what we describe as “off-target” proteins that may also have been in the animal’s blood. When these foreign proteins were injected into patients, it was not uncommon for that patient to experience what is known as “serum sickness” – a condition marked by fever, rash, and/or swollen joints.
Serum sickness plagued the early polyvalent antivenom products in the US. And though some may think otherwise, the truth is that technological advances and improvements in antivenom manufacturing have made treatments much safer and more effective than their predecessors.
Fabulous FABs
The first major innovation in modern antivenom technology was the use of a specific enzyme, papain, to split or cleave the antibody – separating the part of the antibody that specifically attaches to the venom (known as the fragment antigen-binding, or FAB) and the “tail” of the antibody, known as the Fc region (fragment crystallizable region).
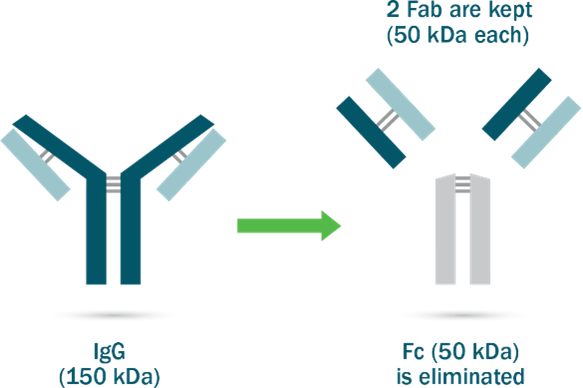
Separating the FABs from the Fc region offers several benefits. It enables FABs to penetrate into tissue inaccessible to larger molecules and allows a large volume of these fragments to swiftly distribute through the body, thereby helping the antivenom neutralize toxins in the tissue.
It also reduces the risk of certain allergic reactions and hypersensitivities, as the Fc region is eliminated and the smaller Fab fragments that remain are cleared from the body rapidly (and so less likely to be recognized by the body’s immune system).
With the FDA’s approval of the first Fab antivenom in 2000, the last whole immunoglobin product was removed from the market.
Only venom-specific antibodies, please
The second major innovation to improve the safety and efficacy of rattlesnake antivenom is a process that ensures only the antibodies that bind to venom are included in the final product.
At BTG Specialty Pharmaceuticals, we accomplish this through a complex manufacturing process called affinity chromatography. This technique isolates and selects only the FABs that bind strongly to a target antigen – in this case venom.
To do so, FABs flow through affinity columns coated with venom. These columns act somewhat like a strainer that drains water while retaining the pasta: in other words, affinity chromatography retains the FAB fragments that bind to target antigens while draining non-specific FAB fragments and other foreign proteins out.
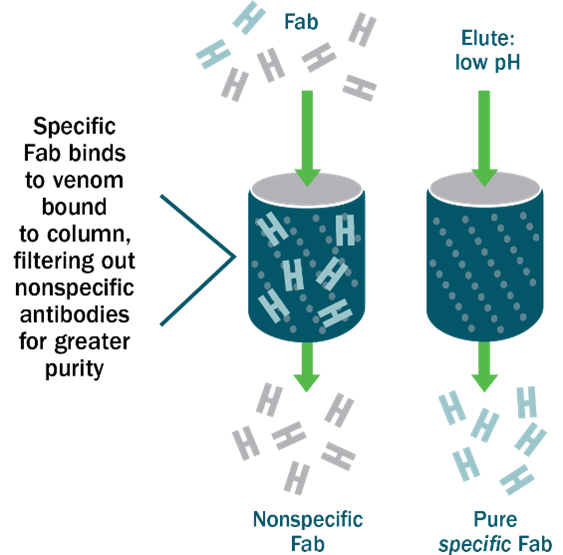
A buffer solution then separates the FAB fragments (the ones that are bound to antigens) from the column –these are the venom-specific antibodies that go into our antivenom. Because every antibody in the finished product is specific to snake venom, CroFab® is less less likely to cause adverse reactions or “serum sickness.”
Every drop of our antivenom product is an antibody fragment, specifically chosen for its ability to bind to venom and small enough to penetrate tissue and neutralize venom’s toxic effects. This helps explain why a recent retrospective study of 1340 CroFab® patients found such a low incidence (1.4%) of acute hypersensitivity reactions.
Cleaving antibodies and affinity chromatography may sound complex. But they’re all part of a manufacturing process designed to yield a high quality product with a lower risk of side effects. The medications they produce are a world away from the older generation of antivenom.
With these scientific and technological improvements, physicians today can be far more confident that the benefits of treating snakebite patients with antivenom far outweigh the risks.